
|
|
back
MENU
|
|
Private
|

Ions
Electrolyte ions
Organic electrolyte conductivity
Solvent Polycarbonate, Acetonitrile (PC, AN)
0.5 – 2.5 M salts concentration
Quaternary Bore or phosphonium BR4- , PR4-
Quaternary ammonium R4N+
Electrode capacitance decreases with the increase of the ion size
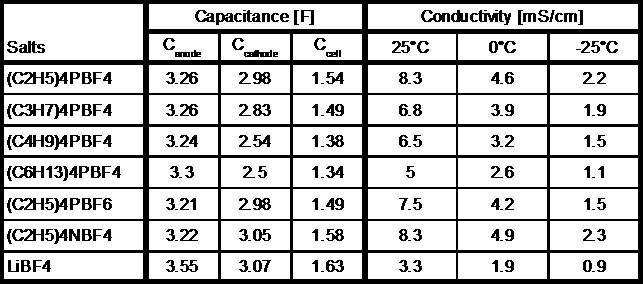
Tetraethyl - ammonium- tetrafluoroborate
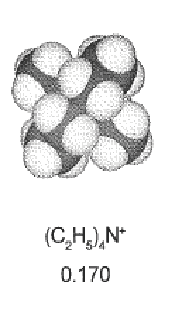
| Negative electrode | Positive electrode |
 |
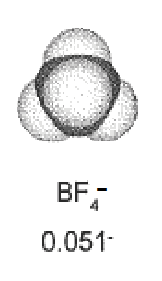 |
| Diameter = 700 pm | Diameter = 460 nm |
Negative ions can go closer from the electrode => higher capacitance.
This is confirmed by cyclic voltammetry
« Electrolyte » resistance as a function of the salt concentration in AN
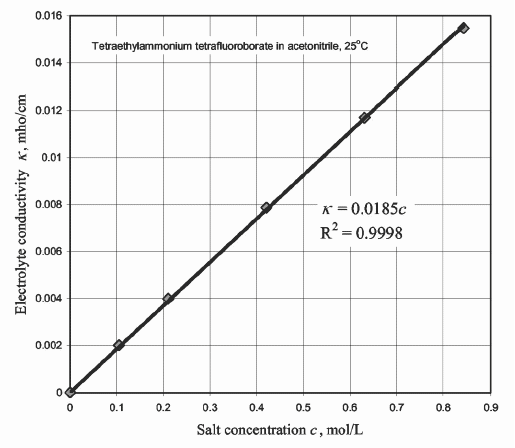
Conductance in mho being the reciprocal of resistance in ohms, mho is ohm spelled backwards!
mho/cm = ohm-1cm-1 = Siemens cm-1